Study design Randomized twosequence twoperiod crossover Treatment sequence is randomized Every subjects gets both treatments Half get generic drug first half get brand drug first minimizesthe sequence effect Crossover design Each subject serves as hisher own control minimizes intersubject variability. Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Bioequivalence study design.
Bioequivalence Study Design, Bioequivalence - Study Design Considerations Study Design Considerations. The test products used in the bioequivalence study must be prepared in accordance with GMP regulations. Conclusion of bioequivalence studies Study design appropri ate and study conduct satisfactory No critical deficiencies or abnormalities methods or statistical analysis Bioequivalence established. A single-dose cross-over design is recommended.
 Pdf The Basic Regulatory Considerations And Prospects For Conducting Bioavailability Bioequivalence Ba Be Studies An Overview Semantic Scholar From semanticscholar.org
Pdf The Basic Regulatory Considerations And Prospects For Conducting Bioavailability Bioequivalence Ba Be Studies An Overview Semantic Scholar From semanticscholar.org
An overview of the generic drug approval process Division of Bioequivalence II Reviewer Kimberly W. This guidance document is being distributed for comment purposes only. The availability of analytical methods 4. Area under the plasma concentration-time curve AUC and maximum plasma concentration Cmax are the.
Containing antacids GMWSI â Bioequivalence Study Information Form Web view in submission of study site normal values for.
Read another article:
The most common designs for bioequivalence studies are replicated crossover nonreplicated crossover and parallel. In other situations bioequivalence may sometimes be demonstrated through comparative clinical trials or pharmacodynamic studies. The test products used in the bioequivalence study must be prepared in accordance with GMP regulations. In vivo comparative bioavailability studies. The availability of analytical methods 4.
 Source: semanticscholar.org
Source: semanticscholar.org
A single-dose cross-over design is recommended. Area under the plasma concentration-time curve AUC and maximum plasma concentration Cmax are the. Biometrical concepts of alternative designs and pooled analysis. Bioequivalence - Study Design Considerations Study Design Considerations. Pdf The Basic Regulatory Considerations And Prospects For Conducting Bioavailability Bioequivalence Ba Be Studies An Overview Semantic Scholar.
 Source: youtube.com
Source: youtube.com
Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Bioequivalence - Study Design Considerations Study Design Considerations. Nature of reference material and dosage form to be tested 3. In a simple parallel study design the statistical analysis should be conducted including the between-subject variability to calculate the 90 confidence interval of the treatment mean difference. Bioavailability And Bioequivalence Study Designs Youtube.
 Source: researchgate.net
Source: researchgate.net
Guidance for the design of bioequivalence studies. A bioequivalence study single-dose or multi-dose should be crossover in design unless a parallel or other design is more appropriate for valid scientific reasons. The test products used in the bioequivalence study must be prepared in accordance with GMP regulations. Based on pharmacokinetic PK data collected bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. Design Of The Four Bioequivalence Studies Download Scientific Diagram.
 Source: researchgate.net
Source: researchgate.net
This guidance document is being distributed for comment purposes only. Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Bioequivalence - Study Design Considerations Study Design Considerations. Guidance for the design of bioequivalence studies. Representation Of Parallel Study Design Download Scientific Diagram.
 Source: slideplayer.com
Source: slideplayer.com
The EoI includes 200 mg capsules. Presents the recent developments in methodology including population and individual bioequivalence. Study design Randomized twosequence twoperiod crossover Treatment sequence is randomized Every subjects gets both treatments Half get generic drug first half get brand drug first minimizesthe sequence effect Crossover design Each subject serves as hisher own control minimizes intersubject variability. Taking into account the pharmacokinetic properties of Molnupiravir the following guidance with regard to the study design should be taken into account. Interchangeability And Study Design Drs Jan Welink Training Workshop Training Of Be Assessors Kiev October Ppt Download.

Objective The basic design for bioequivalence study is determined by. A single-dose cross-over design is recommended. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. 41 Design conduct and evaluation of bioequivalence studies The number of studies and study design depend on the physico-chemical characteristics of the substance its pharmacokinetic properties and proportionality in composition and should be justified accordingly. Be Bio Equivalence Study Design Basics 2 White Paper.
 Source: researchgate.net
Source: researchgate.net
APIdays Paris 2019 - Innovation scale APIs as Digital Factories New Machi. Biometrical concepts of alternative designs and pooled analysis. A bioequivalence study single-dose or multi-dose should be crossover in design unless a parallel or other design is more appropriate for valid scientific reasons. Presents the recent developments in methodology including population and individual bioequivalence. Study Design Of The Crossover Bioequivalence Study Download Table.
 Source: link.springer.com
Source: link.springer.com
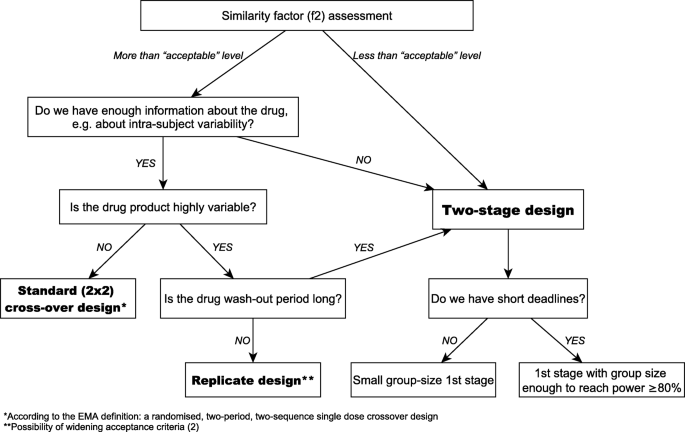
A single-dose cross-over design is recommended. An overview of the generic drug approval process Division of Bioequivalence II Reviewer Kimberly W. 8000-12500 C max AUC 0-t and AUC 0-inf Narrow therapeutic index drug. A bioequivalence study compares the bioavailability between a test and a reference drug product in terms of the rate and extent of drug absorption. 10th Anniversary Of A Two Stage Design In Bioequivalence Why Has It Still Not Been Implemented Springerlink.
 Source: studylib.net
Source: studylib.net
Based on pharmacokinetic PK data collected bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. As recommended by the USFDA in most bioequivalence studies a test drug is compared with the standard reference drug in a group of normal healthy subjects of age 1855 years each receives both the treatments alternately in a crossover fashion two-period two-treatment crossover design with the two phases of treatment separated by a washout period. Presents the recent developments in methodology including population and individual bioequivalence. Taking into account the pharmacokinetic properties of Molnupiravir the following guidance with regard to the study design should be taken into account. Best Bioequivalence Study Template Pekka Heikkila Ceo Statfinn Oy.
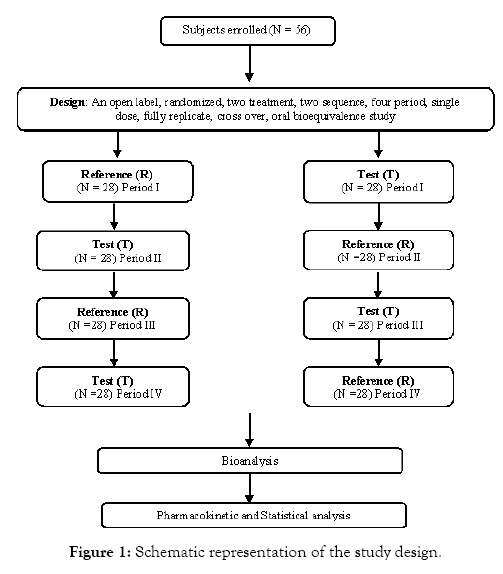 Source: longdom.org
Source: longdom.org
Thus in practice a standard two-sequence two-period or 2x2 crossover design is often applied. Scientific question and objectives to be answered 2. The EoI includes 200 mg capsules. The most common designs for bioequivalence studies are replicated crossover nonreplicated crossover and parallel. A Full Replicate In Vivo Bioequivalence Study Of Two Idelalisib 150 Mg Tablets In Fasted Healthy Adult Human Subjects.
 Source: slideshare.net
Source: slideshare.net
In bioequivalence studies the study design also determines the appropriate statistical model for data analysis. In a simple parallel study design the statistical analysis should be conducted including the between-subject variability to calculate the 90 confidence interval of the treatment mean difference. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. Presents the recent developments in methodology including population and individual bioequivalence. Bioequivalence Study Design.
 Source: semanticscholar.org
Source: semanticscholar.org
Taking into account the pharmacokinetic properties of Molnupiravir the following guidance with regard to the study design should be taken into account. A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects ie each subject is at hisher own control. Bioequivalence - Study Design Considerations Study Design Considerations. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. Ethical Guidelines And Study Design For Bioavailability And Bioequivalence Study Semantic Scholar.
 Source: slideshare.net
Source: slideshare.net
Conclusion of bioequivalence studies Study design appropri ate and study conduct satisfactory No critical deficiencies or abnormalities methods or statistical analysis Bioequivalence established. Presents the recent developments in methodology including population and individual bioequivalence. The availability of analytical methods 4. A bioequivalence study compares the bioavailability between a test and a reference drug product in terms of the rate and extent of drug absorption. Bioavilability And Bioequivalence Study Designs.
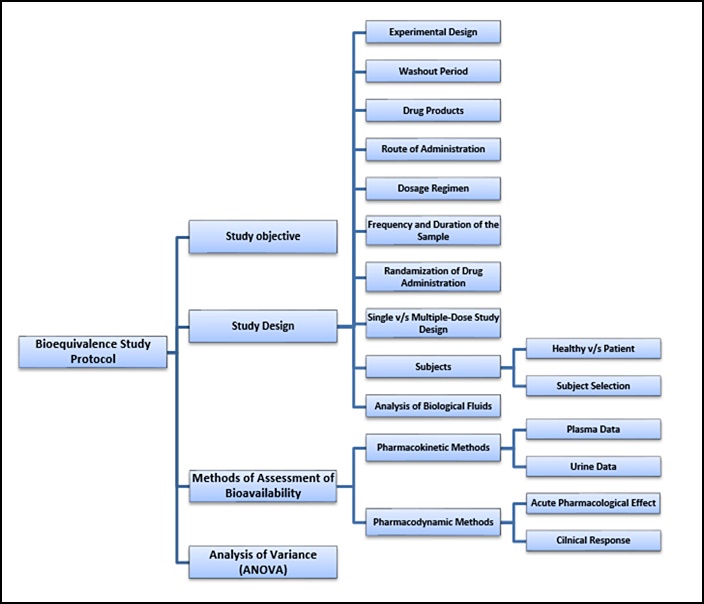 Source: 14impressions.in
Source: 14impressions.in
In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. Guidance for the design of bioequivalence studies. Nature of reference material and dosage form to be tested 3. In a parallel design each subject receives only one formulation in randomized fashion whereas in a crossover design each subject receives different formulations in different time periods. Elements Of Bioequivalence Study Protocol.
 Source: slideshare.net
Source: slideshare.net
Bioequivalence Studies With Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA Guidance for Industry. 90 CI of mean TR. APIdays Paris 2019 - Innovation scale APIs as Digital Factories New Machi. This guidance document is being distributed for comment purposes only. Bioequivalence Study Design.







